Chapter Eighteen: Metabolic Alkalosis, part 1
We are a bit slappy at the beginning of the episode since we had just recorded our conversation with the Glaucomfleckens.
References
Chapter 18 Metabolic alkalosis!
Part 1 February 23, 2023
It is chloride depletion alkalosis, not contraction alkalosis classic review by Galla and Luke, the metabolic alkalosis mavens who review the role of chloride.
On the mechanism by which chloride corrects metabolic alkalosis in man and this is the study when they induced a metabolic alkalosis and studied the effect of treating with KCl vs NaPhos and found the former (with chloride) reversed the alkalosis but not the sodium containing protocol.
Some elegant reports on the increased proximal reabsorption of bicarbonate above normal stimulated by Ang II.
THE RENAL REGULATION OF ACID-BASE BALANCE IN MAN. III. THE REABSORPTION AND EXCRETION OF BICARBONATE 1949 this is the correct figure for 11.14 and shows what happens when filtered bicarb exceeds normal threshold in normal human (men) and appears in the urine.
Masterful review Symposium on acid-base homeostasis. The generation and maintenance of metabolic alkalosis by Seldin and Rector
And a modern review from Michael Emmet! Metabolic Alkalosis - PMC (so many favorite reviews on this exciting topic!) and this one from Soleimani Metabolic Alkalosis Pathogenesis, Diagnosis, and Treatment: Core Curriculum 2022 both of these elaborate on pendrin’s role.
The effect of prolonged administration of large doses of sodium bicarbonate in man (Clin Sci. 1954 Aug;13(3):383-401)
Plus: We got a little off topic and discussed the Kidney Failure Risk Equation: https://kidneyfailurerisk.com/
Outline: Chapter 18 Metabolic Alkalosis
Elevation of arterial pH, increased plasma HCO3, and compensatory hypoventilation
High HCO3 may be compensatory for respiratory acidosis
HCO3 > 40 indicates metabolic alkalosis
Pathophysiology: Two Key Questions
How do patients become alkalotic?
Why do they remain alkalotic?
Generation of Metabolic Alkalosis
Loss of H+ ions
GI loss: vomiting, GI suction, antacids
Renal loss: diuretics, mineralocorticoid excess, hypercalcemia, post-hypercapnia
Administration of bicarbonate
Transcellular shift
K+ loss → H+ shifts intracellularly
Intracellular acidosis
Refeeding syndrome
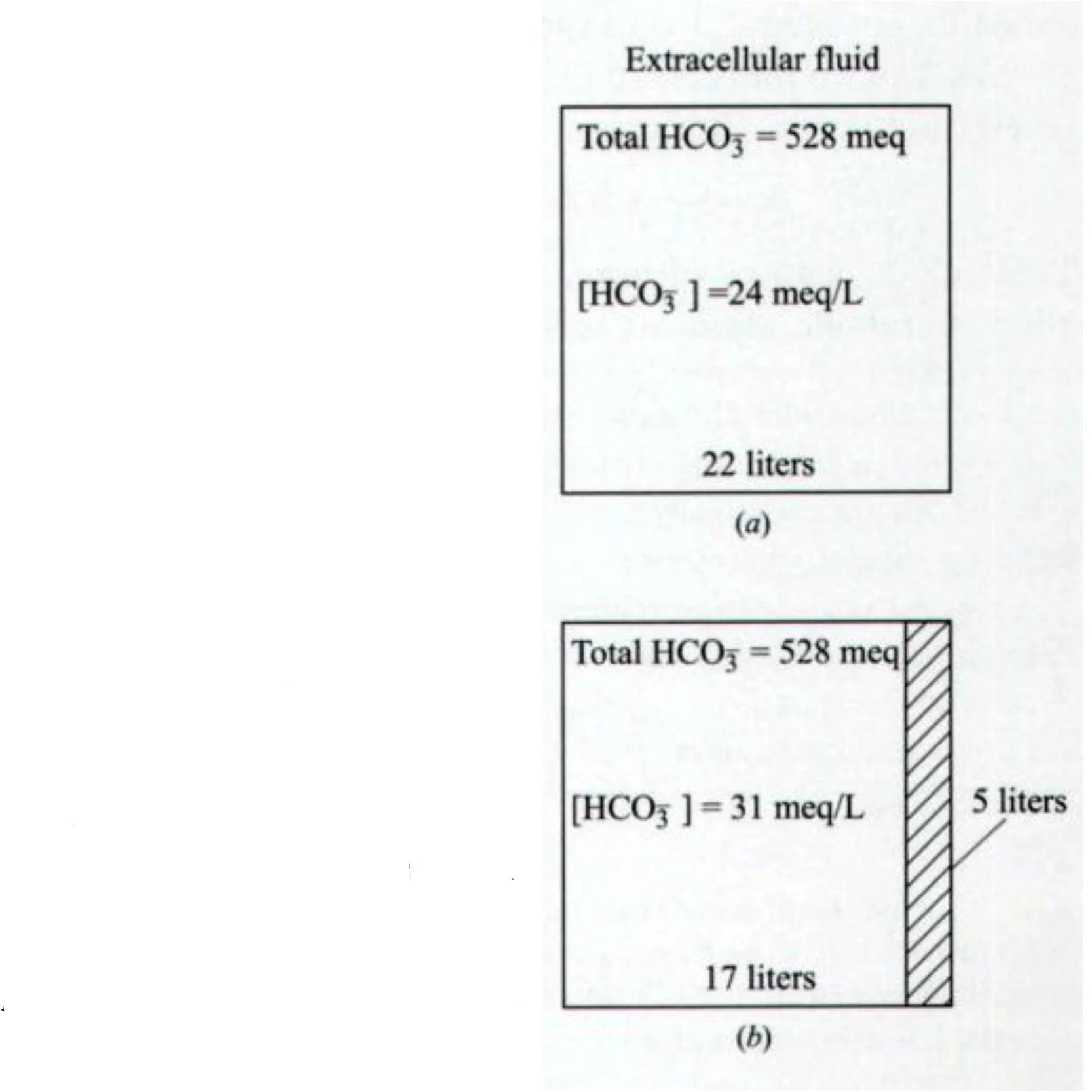
Contraction alkalosis
Same HCO3, smaller extracellular volume → increased [HCO3]
Seen in CF (sweating), illustrated in Fig 18-1
Common theme: hypochloremia is essential for maintenance
Maintenance of Metabolic Alkalosis
Kidneys normally excrete excess HCO3
Example: Normal subjects excrete 1000 mEq NaHCO3/day with minor pH change
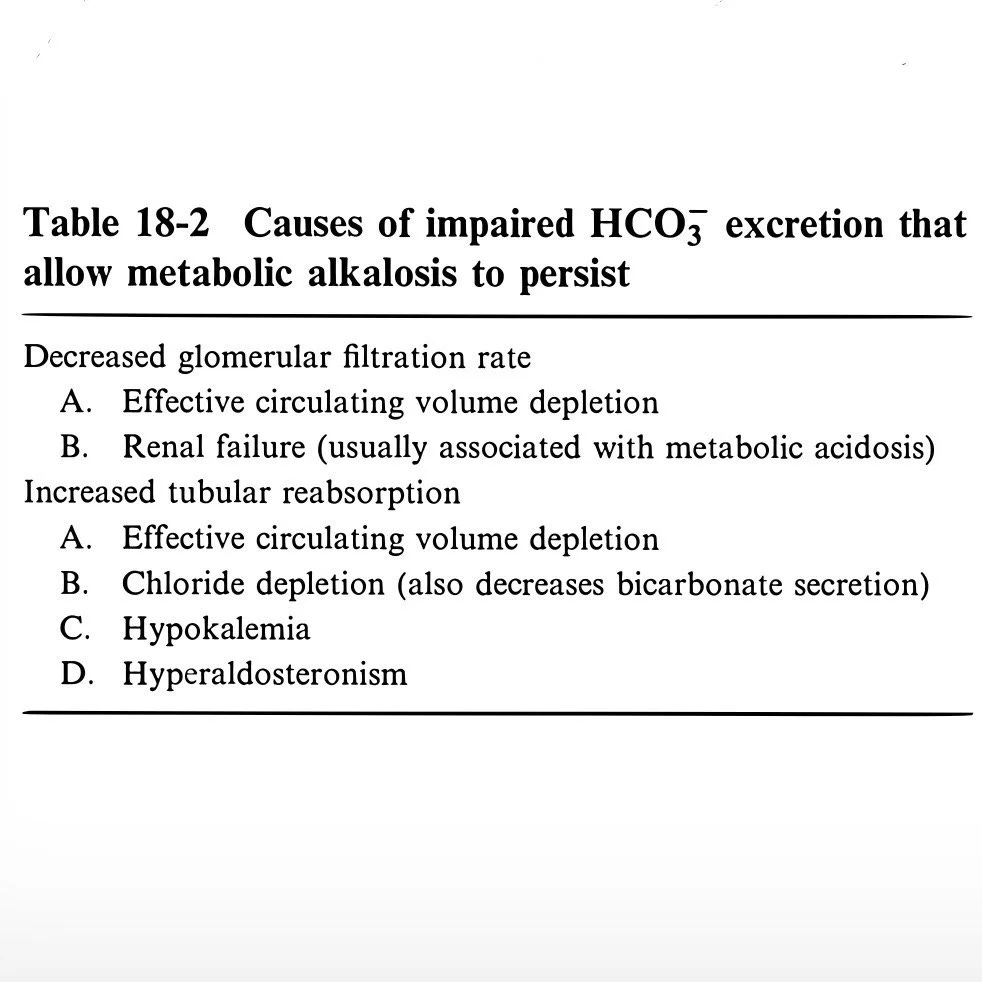
Impaired HCO3 excretion required for maintenance
Table 18-2
Mechanisms of Maintenance
Decreased GFR (less important)
Increased tubular reabsorption
Proximal tubule (PT): reabsorbs 90% of filtered HCO3
TALH and distal nephron manage the rest
Contributing factors:
Effective circulating volume depletion
Enhances HCO3 reabsorption
Ang II increases Na-H exchange
Increased tubular [HCO3] enables more H+ secretion
Distal nephron HCO3 reabsorption
Stimulated by aldosterone (↑ H-ATPase, ↑ Na reabsorption)
Negative luminal charge impedes H+ back-diffusion
Chloride depletion
Reduces NaK2Cl activity → ↑ renin → ↑ aldosterone
Luminal H-ATPase co-secretes Cl → low Cl increases H+ secretion
Cl-HCO3 exchanger needs Cl gradient → low Cl impairs HCO3 secretion
Key conclusion: Cl depletion > volume depletion in perpetuating alkalosis
Albumin corrects volume but not alkalosis
Non-N Cl salts correct alkalosis without fixing volume
Hypokalemia
Stimulates H+ secretion and HCO3 reabsorption
Transcellular shift (H/K exchange) → intracellular acidosis
H-K ATPase reabsorbs K and secretes H
Severe hypokalemia reduces Cl reabsorption → ↑ H+ secretion
Important with mineralocorticoid excess
Respiratory Compensation
Hypoventilation: 0.7 mmHg PCO2 ↑ per 1 mEq/L HCO3 ↑
PCO2 can exceed 60
Rise in PCO2 increases acid excretion (limited effect on pH)
Epidemiology
GI Hydrogen Loss
Gastric juice: high HCl, low KCl
Stomach H+ generation → blood HCO3
Normally recombine in duodenum
Vomiting/antacids prevent recombination → alkalosis
Antacids (e.g., MgOH)
Mg binds fats, leaves HCO3 unbound → alkalosis
Renal failure impairs excretion
Cation exchange resins (SPS, MgCO3) → same effect
Congenital chloridorrhea
High fecal Cl-, low pH → metabolic alkalosis
PPI may help by reducing gastric Cl load
Renal Hydrogen Loss
Mineralocorticoid excess & hypokalemia
Aldosterone → H+ ATPase stimulation, Na+ reabsorption → negative lumen → ↑ H+ secretion
Diuretics (loop/thiazide)
Volume contraction
Secondary hyperaldosteronism
Increased distal flow and H+ loss
Posthypercapnic alkalosis
Chronic respiratory acidosis → ↑ HCO3
Rapid correction (ventilation) → unopposed HCO3 → alkalosis
Gradual CO2 correction needed
Maintenance: hypoxemia, Cl loss
Low chloride intake (infants)
Na+ reabsorption must exchange with H+/K+
H+ co-secretion with Cl impaired if Cl is low
High dose carbenicillin
High Na+ load without Cl
Nonresorbable anion → hypokalemia, alkalosis
Hypercalcemia
↑ Renal H+ secretion & HCO3 reabsorption
Can contribute to milk-alkali syndrome
Rarely causes acidosis via reduced proximal HCO3 reabsorption
Intracellular H+ Shift
Hypokalemia
Common cause and effect of metabolic alkalosis
H+/K+ exchange → intracellular acidosis → ↑ H+ excretion
Refeeding Syndrome
Rapid carb reintroduction → cellular shift
No volume contraction or acid excretion increase
Retention of Bicarbonate
Requires impaired excretion to become significant
Organic anions (lactate, acetate, citrate, ketoacids)
Metabolism → CO2 + H2O + HCO3
Citrate in blood transfusion (16.8 mEq/500 mL)
8 units → alkalosis risk
CRRT + citrate anticoagulant
Sodium bicarbonate therapy
Rebound alkalosis possible with acid reversal (e.g., ketoacidosis)
Extreme cases: pH up to 7.9, HCO3 up to 70
Contraction Alkalosis
NaCl and water loss without HCO3
Seen in vomiting, diuretics, CF sweat
Mild losses neutralized by intracellular buffers
Symptoms
Often asymptomatic
From volume depletion: dizziness, weakness, cramps
From hypokalemia: polyuria, polydipsia, weakness
From alkalosis (rare): paresthesias, carpopedal spasm, lightheadedness
More common in respiratory alkalosis due to rapid pH shift across BBB
Physical exam not usually helpful
Clues: signs of vomiting
Diagnosis
History is key
If unclear, suspect:
Surreptitious vomiting
CF
Secret diuretic use
Mineralocorticoid excess
Use urine chloride
Table 18-3: urine Na is misleading in alkalosis
Table 18-4: urine chemistry changes with complete HCO3 reabsorption
Vomiting: low urine Na, K, Cl + acidic urine
Sufficient NaCl intake prevents this stage
Exceptions to low urine Cl:
Severe hypokalemia
Tubular defects
CKD
Distinguishing from respiratory acidosis
Use pH as guide
Caution with typo (duplicate pCO2)
A-a gradient might help
Treatment
Correct K+ and Cl− deficiency → kidneys self-correct
Upper GI losses: add H2 blockers
Saline-responsive alkalosis
Treat with NaCl
Mechanisms:
Reverse contraction component
Reduce Na+ retention → promote NaHCO3 excretion
↑ distal Cl delivery → enable HCO3 secretion via pendrin
Monitor urine pH: from 5.5 → 7–8 with therapy
Give K+ with Cl, not phosphate, acetate, or bicarbonate
Saline-resistant alkalosis
Seen in edematous states or K+ depletion
Edema (CHF, cirrhosis): use acetazolamide, HCl, dialysis
Acetazolamide: may ↑ CO2 via RBC carbonic anhydrase inhibition
Mineralocorticoid excess: K+ + K-sparing diuretic (use caution)
Severe hypokalemia:
eNaC Na+ reabsorption must be countered by H+ if no K+
Corrects rapidly with K+ replacement
Restores saline responsiveness
Renal failure: requires dialysis