Chapter Seventeen: Introduction to Simple and Mixed Acid-Base Disorders
Is there anything better to a nephrologist than a juicy acid-base problem?
References
I said I used MDCalc but I was mistaken I use MedCalX which is nice but getting dated.
We talked about this out of print book that we love: Cohen, J. J., Kassirer, J. P. (1982). Acid-base. United States: Little, Brown.
Josh mentioned this article that looked at over 17,000 samples with simultaneous measured and calculated bicarbonate and found a very small difference. Comparison of Measured and Calculated Bicarbonate Values | Clinical Chemistry | Oxford Academic
Base deficit or excess- Diagnostic Use of Base Excess in Acid–Base Disorders | NEJM (check out the accompanying letter to the editor from Melanie challenging this article! Along with colleagues Lecker and Zeidel Diagnostic Use of Base Excess in Acid-Base Disorders )
Melanie loves this paper which shows a nice correlation between arterial and venous pH but the rest of the comparisons are disappointing - Comparison of arterial and venous pH, bicarbonate, Pco2 and Po2 in initial emergency department assessment - PMC
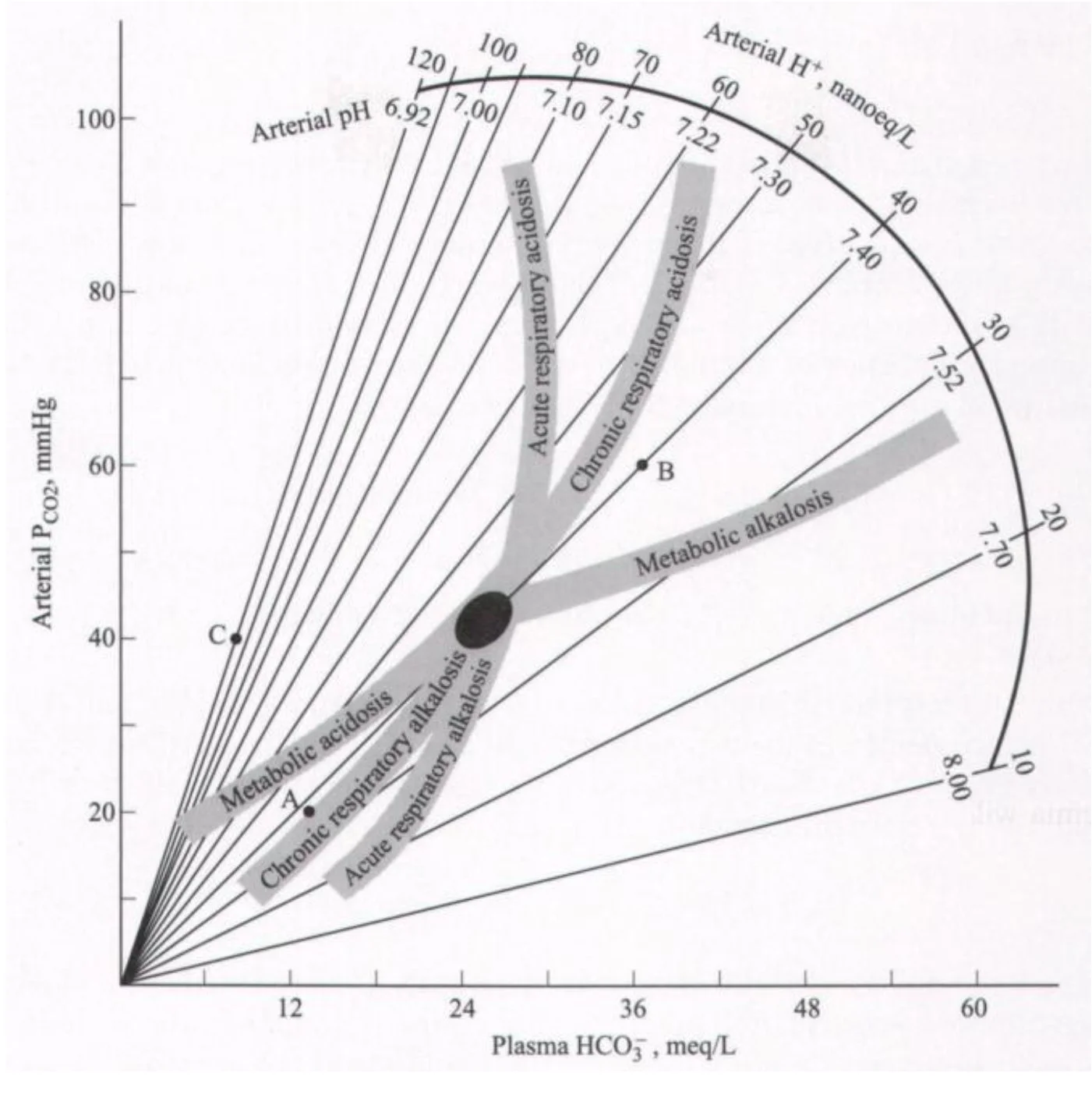
A nomogram for the interpretation of acid-base data is figure 17-1 in the book, this manuscript with the ! in the conclusion creates the acid-base map.
We debated about whether we like Winter’s formula: Quantitative displacement of acid-base equilibrium in metabolic acidosis (melanie does b/c it used real patients).
Amy’s Voice of God on Dietary Acid Load
Review of dietary acid load: https://pubmed.ncbi.nlm.nih.gov/23439373/, https://pubmed.ncbi.nlm.nih.gov/38282081/, https://pubmed.ncbi.nlm.nih.gov/33075387/
Survey data from kidney stone formers regarding sources of dietary acid load: https://pubmed.ncbi.nlm.nih.gov/35752401/
Urine profile for vegans and omnivories (urine pH and cations/anions): https://pubmed.ncbi.nlm.nih.gov/36364731/
SWAP-MEAT pilot trial: https://pubmed.ncbi.nlm.nih.gov/39514692/ looked at urine profile on plant based meat diet (Beyond Meat) versus animal based meat diet
Not all plant meat substitutes are the same in terms of net acid load: https://pubmed.ncbi.nlm.nih.gov/38504022/
Frassetto paper showing that the dietary acid load effect is mostly from sodium chloride: https://pubmed.ncbi.nlm.nih.gov/17522265/
Healthy eating is probably more important than plant based diet for CKD: https://pubmed.ncbi.nlm.nih.gov/37648119/, https://pubmed.ncbi.nlm.nih.gov/32268544/
KDIGO 2024 guidelines: https://kdigo.org/guidelines/ckd-evaluation-and-management/
Association (or lack thereof) of a pro-vegetarian diet and sarcopenia/protein energy wasting in CKD: https://pubmed.ncbi.nlm.nih.gov/39085942/
Outline Chapter 17 Introduction to simple and mixed acid-base disorders
Introduction to Simple and Mixed Acid-Base Disorders
Disturbances of acid-base homeostasis are common clinical problems
Discussed in Chapters 18-21
This chapter reviews:
Basic principles of acid-base physiology
Mechanisms of abnormalities
Evaluation of simple and mixed acid-base disorders
Acid-Base Physiology
Free hydrogen is maintained at a very low concentration
40 nanoEq/L
1 millionth the concentration of Na, K, Cl, HCO3
H+ is highly reactive and must be kept at low concentrations
Compatible H concentration: 16 to 160 nanoEq/L
pH range: 7.8 to 6.8
Buffers prevent excessive variation in H concentration
Most important buffer: HCO3
Reaction: H+ + HCO3 <=> H2CO3 <=> H2O + CO2
H2CO3 exists at low concentration compared to its products
Henderson-Hasselbalch Equation (HH Equation)
Understanding acid-base can use both H+ concentration and pH
Measurement of pH
Must be measured anaerobically to prevent CO2 loss
Measurement methods:
pH: Electrode permeable to H+
PCO2: CO2 electrode
HCO3: Calculated using HH Equation
Alternative: Add strong acid, measure CO2 released
PCO2 * 0.03 gives mEq of CO2
Measured vs. Calculated HCO3
pKa of 6.1 and PCO2 coefficient (0.03) vary
Measurement of CO2 prone to error
Debate remains unresolved
Differences affect anion gap calculations
Arterial vs. Venous Blood Gas (ABG vs. VBG)
Venous pH is lower due to CO2 retention
Venous blood may be as accurate as arterial for pH if well perfused
Pitfalls in pH Measurement
Must cool ABG quickly to prevent glycolysis
Air bubbles affect gas readings
Heparin contamination lowers pH
Arterial pH may not reflect tissue pH
Reduced pulmonary blood flow skews results
End tidal CO2 > 1.5% indicates adequate venous return
Regulation of Hydrogen Concentration
HCO3/CO2 as the Principal Buffer
High HCO3 concentration
Independent regulation of HCO3 (renal) and PCO2 (lungs)
Renal Regulation of HCO3
H secretion reabsorbs filtered bicarbonate
Loss of HCO3 in urine equates to H retention
H combines with NH3 or HPO4, forming new HCO3
Pulmonary Regulation of CO2
CO2 is not an acid but forms H2CO3
Lungs excrete 15,000 mmol of CO2 daily
Kidneys excrete 50-100 mmol of H daily
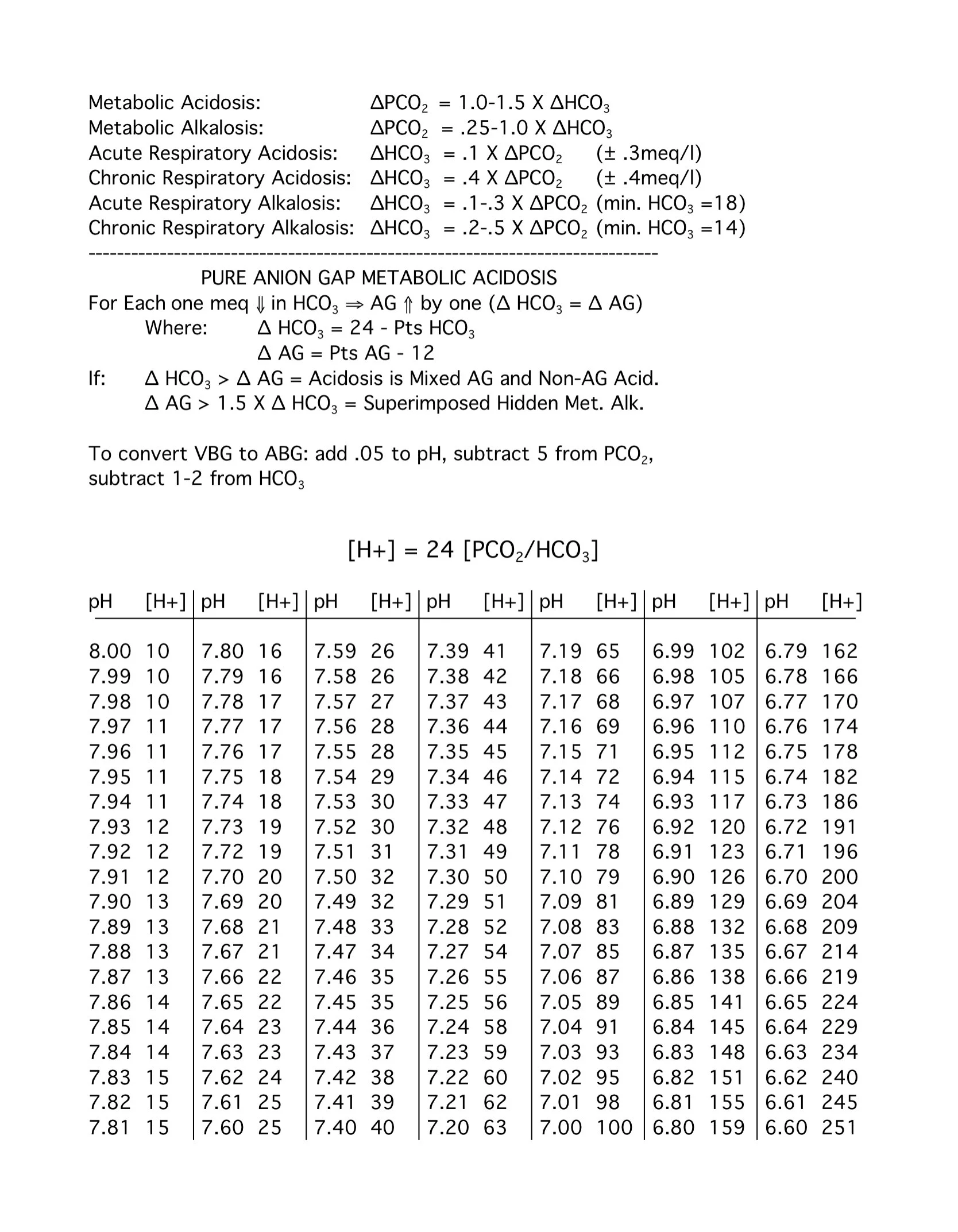
H = 24 * (PCO2 / HCO3)
pH compensation via respiratory and renal adjustments
Acid-Base Disorders
Definitions
Acidemia: Decreased blood pH
Alkalemia: Increased blood pH
Acidosis: Process lowering pH
Alkalosis: Process raising pH
Primary PCO2 abnormalities: Respiratory disorders
Primary HCO3 abnormalities: Metabolic disorders
Compensation moves in the same direction as the primary disorder
Diagnosis requires extracellular pH measurement
Metabolic Acidosis
Low HCO3 and low pH
Causes:
HCO3 loss (e.g., diarrhea)
Buffering of non-carbonic acid (e.g., lactic acid, sulfuric acid in renal failure)
Compensation: Increased ventilation lowers PCO2
Renal excretion of acid restores pH over days
Metabolic Alkalosis
High HCO3 and high pH
Causes:
HCO3 administration
H loss (e.g., vomiting, diuretics)
Compensation: Hypoventilation
Renal HCO3 excretion corrects pH unless volume or chloride depleted
Respiratory Acidosis
Due to decreased alveolar ventilation, increasing PCO2
Compensation: Increased renal H excretion raises HCO3
Acute phase: Large pH drop, small HCO3 increase
Chronic phase: Small pH drop, large HCO3 increase
Respiratory Alkalosis
Due to hyperventilation, reducing CO2 and raising pH
Compensation: Decreased renal H secretion, leading to bicarbonaturia
Time-dependent compensation (acute vs. chronic phases)
Mixed Acid-Base Disorders
Multiple primary disorders can coexist
Example:
Low arterial pH with:
Low HCO3 → Metabolic acidosis
High PCO2 → Respiratory acidosis
Combination indicates mixed disorder
Extent of renal and respiratory compensation clarifies diagnosis
Compensation does not fully restore pH
Example: pH 7.4, PCO2 60, HCO3 36 → Combined respiratory acidosis & metabolic alkalosis
Acid-Base Map illustrates normal responses to disturbances
Clinical Use of Hydrogen Concentration
H+ vs. pH Relationship
H = 24 * (PCO2 / HCO3)
Normal HCO3 cancels out 24, so H = 40 nMol/L
pH to H conversion:
Increase pH by 0.1 → Multiply H by 0.8
Decrease pH by 0.1 → Multiply H by 1.25
Example: Salicylate Toxicity
7.32 / 30 / xx / 15
Goal: Alkalinize urine to pH 7.45 (H+ = 35 nMol/L)
Bicarb needs to reach 20 for compensation
Potassium Balance in Acid-Base Disorders
Metabolic Acidosis
H+ buffered in cells, causing K+ to move extracellularly
K+ rises ~0.6 mEq/L per 0.1 pH drop
Less predictable in lactic or ketoacidosis
DKA-associated hyperkalemia due to insulin deficiency
Hyperkalemia can induce mild metabolic acidosis
Respiratory Acid-Base Disorders
Minimal effect on potassium levels